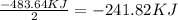Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1

You know the right answer?
Water forms according to the equation below: 2h2(g) + o2(g) jpg 2h2o(g) hrxn = -483.64 kj how much...
Questions


Health, 14.01.2021 23:30


Social Studies, 14.01.2021 23:30






Mathematics, 14.01.2021 23:30



Computers and Technology, 14.01.2021 23:30

Mathematics, 14.01.2021 23:30

Mathematics, 14.01.2021 23:30

Mathematics, 14.01.2021 23:30


English, 14.01.2021 23:30

Mathematics, 14.01.2021 23:30

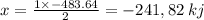

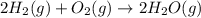
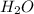 released amount of energy = -483.64 KJ
released amount of energy = -483.64 KJ