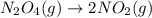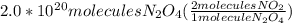Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
How many molecules of no2 are produced when 2.0 × 1020 molecules of n2o4 are decomposed according to...
Questions


Mathematics, 22.08.2021 17:10

World Languages, 22.08.2021 17:10

Mathematics, 22.08.2021 17:10

English, 22.08.2021 17:10


Mathematics, 22.08.2021 17:10




Social Studies, 22.08.2021 17:10



Mathematics, 22.08.2021 17:10




Mathematics, 22.08.2021 17:10

Mathematics, 22.08.2021 17:10

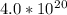 molecules.
molecules.