
Type the correct answer in the box. express the answer to three significant figures. given: ch4 + 2o2 → co2 + 2h2o, δh = -890 kj/mol how much energy is released when 59.7 grams of methane (ch4) reacts with oxygen? the combustion of 59.7 grams of methane releases 34.5 kilojoules of energy.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
You know the right answer?
Type the correct answer in the box. express the answer to three significant figures. given: ch4 + 2...
Questions

Mathematics, 28.09.2020 21:01

History, 28.09.2020 21:01


Social Studies, 28.09.2020 21:01


Mathematics, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01






English, 28.09.2020 21:01

Biology, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01


Mathematics, 28.09.2020 21:01

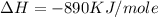
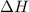 is in negative that means the energy is releasing.
is in negative that means the energy is releasing.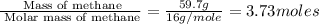
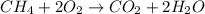
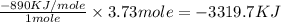 of energy
of energy

