
Chemistry, 02.07.2019 13:30 caitybugking
How do the outer shell electron configurations for ions of group 1,group 2, and group 15 how did the outer shell electron configurations for ions of group 1 group 2 and group 15 group 16 and group 17 elements compared with those of the noble gases

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
How do the outer shell electron configurations for ions of group 1,group 2, and group 15 how did the...
Questions

Mathematics, 10.03.2021 05:20

Mathematics, 10.03.2021 05:20






Mathematics, 10.03.2021 05:20

English, 10.03.2021 05:20

Geography, 10.03.2021 05:20


Mathematics, 10.03.2021 05:20


Mathematics, 10.03.2021 05:20

Mathematics, 10.03.2021 05:20

Mathematics, 10.03.2021 05:20

English, 10.03.2021 05:20


Biology, 10.03.2021 05:20

Social Studies, 10.03.2021 05:20

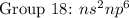 ( have 8 electrons in the outer shell)
( have 8 electrons in the outer shell)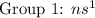 ( have 1 electron in the outer shell}
( have 1 electron in the outer shell}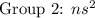 ( have 2 electrons in the outer shell)
( have 2 electrons in the outer shell)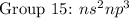 ( have 5 electrons in the outer shell)
( have 5 electrons in the outer shell)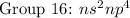 ( have 6 electrons in the outer shell)
( have 6 electrons in the outer shell)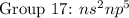 ( have 7 electrons in the outer shell)
( have 7 electrons in the outer shell)

