
Chemistry, 02.07.2019 15:30 Jordan0423
How many moles of potassium chloride and oxygen can be produced from 100.0g of potassium chlorate? the balanced equation is: 2 kclo3=2kcl+3o2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2
You know the right answer?
How many moles of potassium chloride and oxygen can be produced from 100.0g of potassium chlorate?...
Questions




Biology, 04.05.2020 22:32

Mathematics, 04.05.2020 22:32


Mathematics, 04.05.2020 22:32





English, 04.05.2020 22:32

Health, 04.05.2020 22:32


Mathematics, 04.05.2020 22:32

Health, 04.05.2020 22:32


Mathematics, 04.05.2020 22:32

English, 04.05.2020 22:32

Social Studies, 04.05.2020 22:32

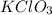 = 100.0 g
= 100.0 g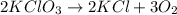
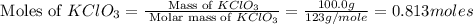
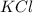 and
and 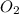 .
.

