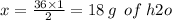Chemistry, 02.07.2019 17:00 lovelylid6969
According to the following equation: 2h2 + o2 → 2h2o how many grams of water will be produced from 1.0 mol hydrogen gas? 34.0 g h2o

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

You know the right answer?
According to the following equation: 2h2 + o2 → 2h2o how many grams of water will be produced from...
Questions

Computers and Technology, 05.03.2020 08:36

Computers and Technology, 05.03.2020 08:36


Physics, 05.03.2020 08:36



Computers and Technology, 05.03.2020 08:37

Mathematics, 05.03.2020 08:37

Advanced Placement (AP), 05.03.2020 08:37


Spanish, 05.03.2020 08:37









Mathematics, 05.03.2020 08:39