
Chemistry, 02.07.2019 17:00 2018jecr46871
You discover a chemical reaction that produces 1 moles of valuable propane for every 6 moles of carbon supplied. essentially, this is an exchange rate of 1 moles of propane for every 6 moles of carbon. if you have 1.8 moles of carbon, how many moles of propane can you produce using this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3
You know the right answer?
You discover a chemical reaction that produces 1 moles of valuable propane for every 6 moles of carb...
Questions






Engineering, 14.06.2021 17:30

English, 14.06.2021 17:30



Mathematics, 14.06.2021 17:30


Mathematics, 14.06.2021 17:30

History, 14.06.2021 17:30

Mathematics, 14.06.2021 17:30


Mathematics, 14.06.2021 17:30


Spanish, 14.06.2021 17:30

Mathematics, 14.06.2021 17:30

Mathematics, 14.06.2021 17:30

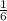 ×1.8 = 0.3 moles of the propane can be prepared.
×1.8 = 0.3 moles of the propane can be prepared.

