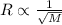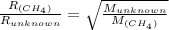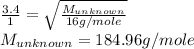Chemistry, 02.07.2019 23:00 timothyashburn8
Explain the relationship between the rate of effusion of a gas and its molar mass. methane gas (ch4) effuses 3.4 times faster than an unknown gas. determine the molar mass of the unknown gas. show your work or explain your answer, giving specific values used to determine the answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3


Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Explain the relationship between the rate of effusion of a gas and its molar mass. methane gas (ch4)...
Questions

Mathematics, 17.05.2021 20:50


Mathematics, 17.05.2021 20:50


Mathematics, 17.05.2021 20:50

Mathematics, 17.05.2021 20:50

Mathematics, 17.05.2021 20:50

English, 17.05.2021 20:50





English, 17.05.2021 20:50



Physics, 17.05.2021 20:50




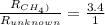
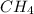 = 16 g/mole
= 16 g/mole