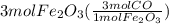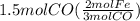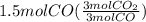Chemistry, 03.07.2019 00:30 ayoismeisalex
Read the chemical equation. fe2o3 + co → fe + co2 if 3 moles of fe2o3 react with 1.5 moles of co, how many moles of each product are formed? a. 1 mole of fe and 1.5 moles of co2 b. 0.5 mole of fe and 1 mole of co2 c.6 moles of fe and 9 moles of co2 d.3 moles of fe and 2 moles of co2 update; its not c

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3
You know the right answer?
Read the chemical equation. fe2o3 + co → fe + co2 if 3 moles of fe2o3 react with 1.5 moles of co, ho...
Questions



Mathematics, 24.12.2019 08:31





Mathematics, 24.12.2019 08:31


History, 24.12.2019 08:31

Physics, 24.12.2019 08:31

History, 24.12.2019 08:31

Mathematics, 24.12.2019 08:31

Mathematics, 24.12.2019 08:31

Chemistry, 24.12.2019 08:31

Mathematics, 24.12.2019 08:31




Mathematics, 24.12.2019 08:31

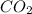 .
.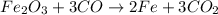
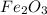 and CO, From given data, 3 moles of
and CO, From given data, 3 moles of