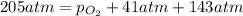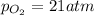Chemistry, 03.07.2019 01:00 FailingstudentXD
Ascuba diver’s air tank contains oxygen, helium, and nitrogen at a total pressure of 205 atmospheres. the partial pressure of nitrogen is 143 atmospheres, and the partial pressure of helium is 41 atmospheres. what is the partial pressure of oxygen in the tank? a. 21 atm b. 103 atm c. 307 atm d. 389 atm

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
Ascuba diver’s air tank contains oxygen, helium, and nitrogen at a total pressure of 205 atmospheres...
Questions



English, 14.01.2020 16:31


Mathematics, 14.01.2020 16:31

Mathematics, 14.01.2020 16:31



Mathematics, 14.01.2020 16:31

Biology, 14.01.2020 16:31

Physics, 14.01.2020 16:31

Mathematics, 14.01.2020 16:31

Biology, 14.01.2020 16:31




Business, 14.01.2020 16:31

English, 14.01.2020 16:31

History, 14.01.2020 16:31

Mathematics, 14.01.2020 16:31

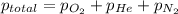
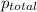 = 205 atm
= 205 atm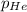 = 41 atm
= 41 atm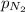 = 143 atm
= 143 atm