
Chemistry, 03.07.2019 03:30 trinity7265
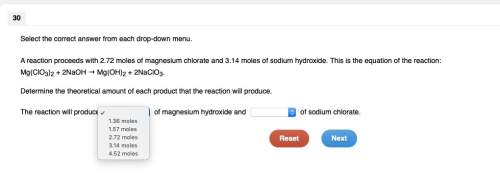
Areaction proceeds with 2.72 moles of magnesium chlorate and 3.14 moles of sodium hydroxide. this is the equation of the reaction: mg(clo3)2 + 2naoh → mg(oh)2 + 2naclo3.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
You know the right answer?
Areaction proceeds with 2.72 moles of magnesium chlorate and 3.14 moles of sodium hydroxide. this is...
Questions

Mathematics, 03.07.2019 10:10


Mathematics, 03.07.2019 10:10


Social Studies, 03.07.2019 10:10

Mathematics, 03.07.2019 10:10

Computers and Technology, 03.07.2019 10:10

Mathematics, 03.07.2019 10:10

Computers and Technology, 03.07.2019 10:10



English, 03.07.2019 10:10


Mathematics, 03.07.2019 10:10

History, 03.07.2019 10:10

Health, 03.07.2019 10:10

SAT, 03.07.2019 10:10

English, 03.07.2019 10:10


Mathematics, 03.07.2019 10:10



