
Chemistry, 03.07.2019 04:00 guzmangisselle
Why are saturated hydrocarbons generally less reactive than unsaturated hydrocarbons? a. atoms are not easily added to molecules with single bonds. b. atoms are not easily added to molecules with double bonds. c. saturated hydrocarbons have fewer carbon atoms than unsaturated hydrocarbons.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
Why are saturated hydrocarbons generally less reactive than unsaturated hydrocarbons? a. atoms are...
Questions

History, 30.10.2019 10:31


History, 30.10.2019 10:31


Mathematics, 30.10.2019 10:31

Physics, 30.10.2019 10:31

Social Studies, 30.10.2019 10:31


Chemistry, 30.10.2019 10:31



Mathematics, 30.10.2019 10:31


Geography, 30.10.2019 10:31

Mathematics, 30.10.2019 10:31

Mathematics, 30.10.2019 10:31


Mathematics, 30.10.2019 10:31


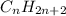
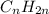 and
and 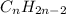
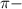 bonds. They are weaker than
bonds. They are weaker than 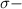 bonds and thus can be easily broken. Addition reactions is more favorable for the compounds having
bonds and thus can be easily broken. Addition reactions is more favorable for the compounds having 

