
Chemistry, 03.07.2019 11:30 lobatospitones
In this reaction, how does the rate of forward reaction vary with the concentration of the product? 2h2s(g) ⇌ 2h2(g) + s2(g) it increases with an increase in the concentration of s2(g). it decreases with a decrease in the concentration of h2(g). it increases with a decrease in the concentration of h2(g). it decreases with an increase in the concentration of s2(g). it decreases with increase in the concentration of h2(g). multiple answers

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
You know the right answer?
In this reaction, how does the rate of forward reaction vary with the concentration of the product?...
Questions


Mathematics, 08.01.2020 00:31

Computers and Technology, 08.01.2020 00:31





French, 08.01.2020 00:31

History, 08.01.2020 00:31



Mathematics, 08.01.2020 00:31

Chemistry, 08.01.2020 00:31




History, 08.01.2020 00:31

Computers and Technology, 08.01.2020 00:31


Mathematics, 08.01.2020 00:31

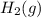 . D) It decreases with increase in the concentration of
. D) It decreases with increase in the concentration of 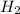 or
or 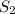 is increased, the reaction will shift in backward direction and the rate of forward direction decreases.
is increased, the reaction will shift in backward direction and the rate of forward direction decreases.

