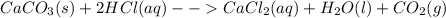The reaction between calcium carbonate (caco3) and hcl produces calcium chloride (cacl2), carbon dioxide (co2), and water (h2o).what happens when the concentration of hydrogen chloride (hcl) molecules is doubled in this reaction? caco3 + 2hcl → cacl2 + co2 + h2o when the hydrogen chloride concentration doubles, the number of collisions between the reactants (increases, decreases, remains constant) , which causes the rate of the forward reaction to (increase, decrease, remain constant)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
The reaction between calcium carbonate (caco3) and hcl produces calcium chloride (cacl2), carbon dio...
Questions


Mathematics, 24.10.2021 14:00

Mathematics, 24.10.2021 14:00


English, 24.10.2021 14:00

Mathematics, 24.10.2021 14:00


Mathematics, 24.10.2021 14:00

Mathematics, 24.10.2021 14:00

Social Studies, 24.10.2021 14:00

Mathematics, 24.10.2021 14:00

Business, 24.10.2021 14:00



History, 24.10.2021 14:00


Mathematics, 24.10.2021 14:00

Mathematics, 24.10.2021 14:00

History, 24.10.2021 14:00