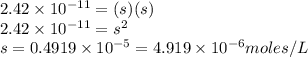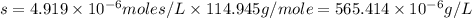Chemistry, 03.07.2019 12:00 taterbuglee2003
The ksp of manganese(ii) carbonate, mnco3, is 2.42 × 10-11. calculate the solubility of this compound in g/l.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
The ksp of manganese(ii) carbonate, mnco3, is 2.42 × 10-11. calculate the solubility of this compoun...
Questions


English, 18.05.2021 20:20

Geography, 18.05.2021 20:20

Mathematics, 18.05.2021 20:20


Mathematics, 18.05.2021 20:20

English, 18.05.2021 20:20


Mathematics, 18.05.2021 20:20

History, 18.05.2021 20:20

Health, 18.05.2021 20:20

Mathematics, 18.05.2021 20:20







Mathematics, 18.05.2021 20:20

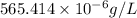 .
.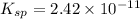
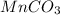 = 114.945g/mole
= 114.945g/mole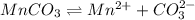
![K_{sp}=[Mn^{2+}][CO^{2-}_3]](/tpl/images/0046/3200/4b0f3.png)