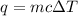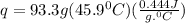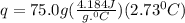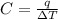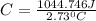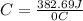Chemistry, 03.07.2019 13:00 xmanavongrove55
Acalorimeter contained 75.0 g of water at 16.95 c. a 93.3-g sample of iron at 65.58 c was placed in it, giving a final temperature of 19.68 c for the system. calculate the heat capacity of the calorimeter. specific heats are 4.184 j/g c for h2o and 0.444 j/g c for fe.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

You know the right answer?
Acalorimeter contained 75.0 g of water at 16.95 c. a 93.3-g sample of iron at 65.58 c was placed in...
Questions



History, 19.08.2019 08:30


Social Studies, 19.08.2019 08:50

History, 19.08.2019 08:50


Social Studies, 19.08.2019 08:50

Mathematics, 19.08.2019 08:50

Mathematics, 19.08.2019 08:50



Mathematics, 19.08.2019 08:50

Chemistry, 19.08.2019 08:50

English, 19.08.2019 08:50

English, 19.08.2019 08:50

Health, 19.08.2019 08:50

Biology, 19.08.2019 08:50

Mathematics, 19.08.2019 08:50

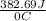 .
. for iron metal = 65.58 - 19.68 = 45.9 degree C
for iron metal = 65.58 - 19.68 = 45.9 degree C