
After the completion of a gas forming reaction, the column of water remaining in the collection flask was measured to me 55mm high, the vapor pressure of water is 0.0313atm at 25oc, and the atmospheric pressure that day was measured as 0.950 atm, what is the partial pressure of the gas produced by the reaction? 1mmhg=13.595mm h2o and 1atm=760mmhg

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1
You know the right answer?
After the completion of a gas forming reaction, the column of water remaining in the collection flas...
Questions


Computers and Technology, 27.08.2020 04:01





English, 27.08.2020 04:01


History, 27.08.2020 04:01



History, 27.08.2020 04:01







Biology, 27.08.2020 04:01

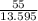 = 4.045 mm of Hg [As 1 mm of Hg is equivalent to 13.595 mm of water]
= 4.045 mm of Hg [As 1 mm of Hg is equivalent to 13.595 mm of water]

