
Chemistry, 03.07.2019 17:30 enitramedouard12
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g); molar mass of butane 58.12g/mol calculate the mass of butane needed to produce 90.9 g of carbon dioxide. calculate the mass of butane needed to produce 90.9 g of carbon dioxide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3


Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2
You know the right answer?
2c4h10(g)+13o2(g)→10h2o(g)+8co2(g); molar mass of butane 58.12g/mol calculate the mass of butane ne...
Questions



Mathematics, 29.04.2021 17:50

Mathematics, 29.04.2021 17:50

English, 29.04.2021 17:50

Mathematics, 29.04.2021 17:50

Advanced Placement (AP), 29.04.2021 17:50


Physics, 29.04.2021 17:50


Social Studies, 29.04.2021 17:50

Mathematics, 29.04.2021 17:50






Biology, 29.04.2021 17:50


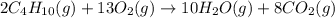
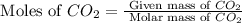
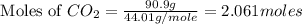
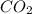 produced from 2 moles
produced from 2 moles 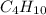
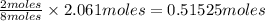 of
of 

