
Chemistry, 03.07.2019 20:30 juan01sebastian00
Cobalt (ii) chloride exits in hydrated forms, the dehydrate and hexahydrate forms. a chemist grabs cobalt (ii) chloride hexahydrate from the shelf and wants to dehydrate the salt. if 5.00g of hexahydrate were heated to drive off waters of hydration, how many grams of the anhydrous salt would remain?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
Cobalt (ii) chloride exits in hydrated forms, the dehydrate and hexahydrate forms. a chemist grabs c...
Questions


Mathematics, 30.10.2020 19:20



Mathematics, 30.10.2020 19:20

Mathematics, 30.10.2020 19:20




English, 30.10.2020 19:20


Chemistry, 30.10.2020 19:20

Mathematics, 30.10.2020 19:20




Biology, 30.10.2020 19:20



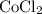 .
.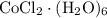 .
.  Chloride- 35.45
Chloride- 35.45 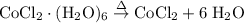
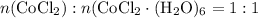 Mass ratio:
Mass ratio: 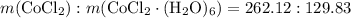
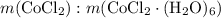 indicates that 262.12 grams of cobalt (II) chloride hexahydrate decomposes to produce 129.83 grams of its corresponding anhydrous salt. Accordingly, heating 5.00 grams of the hexahydrate would produce 2.48 grams of its anhydrate.
indicates that 262.12 grams of cobalt (II) chloride hexahydrate decomposes to produce 129.83 grams of its corresponding anhydrous salt. Accordingly, heating 5.00 grams of the hexahydrate would produce 2.48 grams of its anhydrate.

