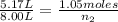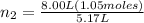Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1
You know the right answer?
An inflatable toy starts with 1.05 moles of air and a volume of 5.17 liters. when fully inflated, th...
Questions





Mathematics, 21.04.2020 16:59


Mathematics, 21.04.2020 16:59


History, 21.04.2020 16:59

Mathematics, 21.04.2020 16:59

Mathematics, 21.04.2020 16:59

Mathematics, 21.04.2020 16:59

Mathematics, 21.04.2020 16:59


Social Studies, 21.04.2020 16:59





History, 21.04.2020 16:59

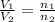
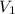 = 5.17 L,
= 5.17 L, 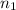 = 1.05 moles
= 1.05 moles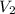 = 8.00 L,
= 8.00 L, 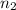 = ?
= ?