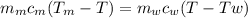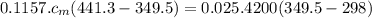Ahot lump of 115.7 g of an unknown substance initially at 168.3°c is placed in 25.0 ml of water initially at 25.0°c and allowed to reach thermal equilibrium. the final temperature of the system is 76.5°c. what is the identity of the unknown substance? assume no heat is lost to the surroundings

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
Ahot lump of 115.7 g of an unknown substance initially at 168.3°c is placed in 25.0 ml of water init...
Questions



Chemistry, 06.11.2020 07:40

History, 06.11.2020 07:40



Spanish, 06.11.2020 07:40


Mathematics, 06.11.2020 07:40


Business, 06.11.2020 07:40

English, 06.11.2020 07:40

Biology, 06.11.2020 07:40


Health, 06.11.2020 07:40


Mathematics, 06.11.2020 07:40



English, 06.11.2020 07:40

 .
.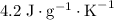 and a density of
and a density of 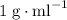 . 25.0 milliliters of water thus has a mass of 25.0 grams.
. 25.0 milliliters of water thus has a mass of 25.0 grams. 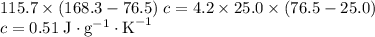
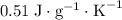 . This substance is thus probably steel.
. This substance is thus probably steel.