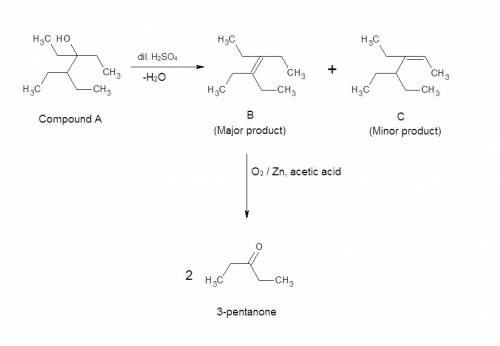
Compound a, c10h22o, undergoes reaction with dilute h2so4 at 50°c to yield a mixture of two alkenes, b and c, c10h20. the major alkene product, b, gives only 3-pentanone after ozone treatment followed by reduction with zinc in acetic acid. draw the structure of the major alkene product, compound b.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3
You know the right answer?
Compound a, c10h22o, undergoes reaction with dilute h2so4 at 50°c to yield a mixture of two alkenes,...
Questions


Health, 28.12.2019 01:31








Social Studies, 28.12.2019 01:31

Computers and Technology, 28.12.2019 01:31









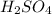 at
at 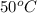 to yield a mixture of two alkenes, B and C. In these two alkenes B is the major product and C is the minor product.
to yield a mixture of two alkenes, B and C. In these two alkenes B is the major product and C is the minor product.