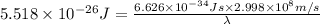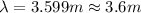Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
Calculate the wavelength of a photon of energy 5.518 × 10-26 joules. (planck's constant is 6.626 × 1...
Questions

Social Studies, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01



Computers and Technology, 02.10.2020 14:01

Social Studies, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01


Mathematics, 02.10.2020 14:01

Spanish, 02.10.2020 14:01


Health, 02.10.2020 14:01

History, 02.10.2020 14:01



Mathematics, 02.10.2020 14:01




Health, 02.10.2020 14:01

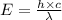
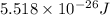
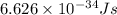
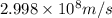
 = wavelength of a photon = ?
= wavelength of a photon = ?