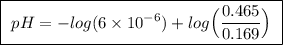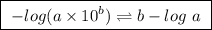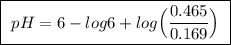Chemistry, 04.07.2019 11:00 rntaran2002
Abuffer solution is made by dissolving 0.45 moles of a weak acid (ha) and 0.33 moles of koh into 710 ml of solution. what is the ph of this buffer? ka = 6x10-6 for ha.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
Abuffer solution is made by dissolving 0.45 moles of a weak acid (ha) and 0.33 moles of koh into 710...
Questions

Computers and Technology, 14.10.2019 22:30

Biology, 14.10.2019 22:30


Physics, 14.10.2019 22:30


Computers and Technology, 14.10.2019 22:30

Mathematics, 14.10.2019 22:30

Mathematics, 14.10.2019 22:30


Chemistry, 14.10.2019 22:30


Mathematics, 14.10.2019 22:30


Chemistry, 14.10.2019 22:30


Spanish, 14.10.2019 22:30


Health, 14.10.2019 22:30

Mathematics, 14.10.2019 22:30

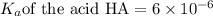
![[concentration]=\frac{\text{number of moles}}{\text{volume in litres}}](/tpl/images/0049/9814/01f13.png)
![[HA]=\frac{0.45}{0.710} mol/L,[KOH]=\frac{0.33}{0.710} mol/L](/tpl/images/0049/9814/a3a14.png)
![pH=pK_a+log\frac{[base]}{[acid]}](/tpl/images/0049/9814/9facd.png) ...(1)
...(1)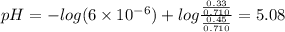
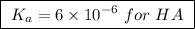
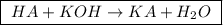
![\boxed{ \ pH = pKa + log_{10} \frac{[A-]}{[HA]} \ }](/tpl/images/0049/9814/90294.png)
![\boxed{ \ [HA] = \frac{0.12 \ moles}{0.710 \ L} = 0.169 \ M \ }](/tpl/images/0049/9814/115c3.png)
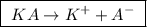 we observe that there is one A⁻ ion or the valence is 1.
we observe that there is one A⁻ ion or the valence is 1. ![\boxed{ \ [A^-] = [KA] \times valence \ }](/tpl/images/0049/9814/39c11.png) , so
, so![\boxed{ \ [A^-] = \frac{0.33 \ moles}{0.710 \ L} \times 1 = 0.465 \ M \ }](/tpl/images/0049/9814/6cb5e.png)