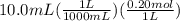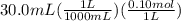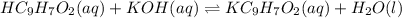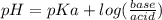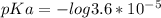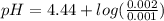Chemistry, 04.07.2019 23:00 dnprops1544
What is the ph when 10.0 ml of 0.20 m potassium hydroxide is added to 30.0 ml of 0.10 m cinnamic acid, hc9h7o2 (ka = 3.6 × 10–5) 4.74 9.26 1.60 12.40

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2

Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1

Chemistry, 23.06.2019 13:00
What type of reaction is this equation c2h5s + o2 > co2 + h2o + so2
Answers: 2
You know the right answer?
What is the ph when 10.0 ml of 0.20 m potassium hydroxide is added to 30.0 ml of 0.10 m cinnamic aci...
Questions



Arts, 06.09.2021 18:10

Mathematics, 06.09.2021 18:10

Mathematics, 06.09.2021 18:10




Mathematics, 06.09.2021 18:10

English, 06.09.2021 18:10


English, 06.09.2021 18:10

Chemistry, 06.09.2021 18:10


English, 06.09.2021 18:10


Arts, 06.09.2021 18:10


Mathematics, 06.09.2021 18:10