
2suppose that a new element (e) were discovered that existed as three natural isotopes: 25% of atoms had a mass of 278, 38% had a mass of 281, and the remained had a mass of 285. what would be listed as the atomic mass of this element? explain your logic and show all steps.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

You know the right answer?
2suppose that a new element (e) were discovered that existed as three natural isotopes: 25% of atom...
Questions

Mathematics, 27.10.2020 21:50

Mathematics, 27.10.2020 21:50

Advanced Placement (AP), 27.10.2020 21:50




Computers and Technology, 27.10.2020 21:50

English, 27.10.2020 21:50



Mathematics, 27.10.2020 21:50

English, 27.10.2020 21:50

Physics, 27.10.2020 21:50


Mathematics, 27.10.2020 21:50

Physics, 27.10.2020 21:50


Mathematics, 27.10.2020 21:50

History, 27.10.2020 21:50

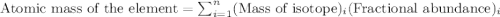 .....(1)
.....(1)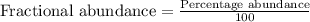
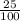 =0.25
=0.25![\text{Atomic mass of the element}=\left[(278\times 0.25)+(281\times 0.38)+(285\times 0.37) \right ]](/tpl/images/0052/4546/3e73a.png)


