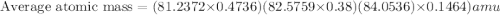Chemistry, 05.07.2019 08:30 aduncan3426
The element loncapium has three isotopes. the first lc-82 has a mass of 81.2372 amu and makes up 47.36% of a standard sample. the second isotope, lc-83, has a mass of 82.5759 amu and makes up 38% of a standard sample. the third isotope, lc-85 has a mass of 84.0536 amu and makes up the remainder of the sample. what is the average atomic mass of this element?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
The element loncapium has three isotopes. the first lc-82 has a mass of 81.2372 amu and makes up 47....
Questions


Physics, 25.01.2021 18:10


Mathematics, 25.01.2021 18:10

Computers and Technology, 25.01.2021 18:10






Mathematics, 25.01.2021 18:10

Chemistry, 25.01.2021 18:10


English, 25.01.2021 18:10

Spanish, 25.01.2021 18:10


Social Studies, 25.01.2021 18:10



Mathematics, 25.01.2021 18:10

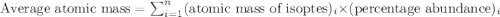 ....(1)
....(1)