
Which of the following laboratory procedures best illustrates the law of conservation of mass? reacting 12 g of c with 32 g of o2 to form 44 g of co2 and unused reactants weighing 100 g of cu powder and 100 g of fe filings heating 100 g of caco3 to produce 56 g of cao dissolving 10 g of nacl in 100 g of water

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
Which of the following laboratory procedures best illustrates the law of conservation of mass? rea...
Questions




Mathematics, 26.01.2022 17:40




Advanced Placement (AP), 26.01.2022 17:40


English, 26.01.2022 17:40






Mathematics, 26.01.2022 17:40



Mathematics, 26.01.2022 17:40

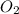 to form 44 g of
to form 44 g of 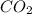 and unused reactants.
and unused reactants.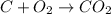
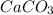 to produce 56 g of CaO
to produce 56 g of CaO



