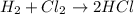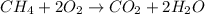Chemistry, 05.07.2019 12:30 izzysmith6836
Identify the type of reaction represented by each equation. a: h2 + cl2 → 2hcl b: ch4 + 2o2 → co2 + 2h2o equation a represents a reaction, and equation b represents reaction. choices for both blanks combustion decombustion synthesis double displacement single displacement choose one for each blank there are some notes and examples in the images my opinions are 1. 2blank--combustion tell me if im correct 16pionts

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2


Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
Identify the type of reaction represented by each equation. a: h2 + cl2 → 2hcl b: ch4 + 2o2 → co2...
Questions



History, 24.04.2020 21:02

Mathematics, 24.04.2020 21:02

Social Studies, 24.04.2020 21:02




Biology, 24.04.2020 21:02

Biology, 24.04.2020 21:02

Mathematics, 24.04.2020 21:02



History, 24.04.2020 21:02

Mathematics, 24.04.2020 21:02

Mathematics, 24.04.2020 21:02

Social Studies, 24.04.2020 21:02


Chemistry, 24.04.2020 21:02