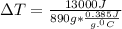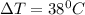Chemistry, 05.07.2019 18:00 calebmoore925
A1.1 kg block of iron at 38 ∘c is rapidly heated by a torch such that 13 kj is transferred to it. what temperature would the block of iron reach assuming the complete transfer of heat and no loss to the surroundings? if the same amount of heat was quickly transferred to a 890 g pellet of copper at 38 ∘c, what temperature would it reach before losing heat to the surroundings? cs, fe(s)= 0.450 j/g*c cs, cu(s)= 0.385 j/g*c

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
A1.1 kg block of iron at 38 ∘c is rapidly heated by a torch such that 13 kj is transferred to it. wh...
Questions

Mathematics, 27.04.2021 21:20


Mathematics, 27.04.2021 21:20

English, 27.04.2021 21:20





History, 27.04.2021 21:20

Mathematics, 27.04.2021 21:20





Mathematics, 27.04.2021 21:20

English, 27.04.2021 21:20




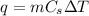
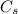 is specific heat and
is specific heat and  is the change in temperature.
is the change in temperature.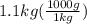 = 1100 g
= 1100 g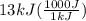 = 13000 J
= 13000 J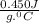
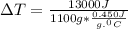
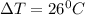
 .
.