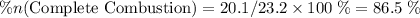Chemistry, 05.07.2019 18:00 amortalstardev
Octane (c8h18) is a component of gasoline. complete combustion of octane yields h2o and co2. incomplete combustion produces h2o and co, which not only reduces the efficiency of the engine using the fuel but is also toxic. in a certain test run, 1.000 gallon (gal) of octane is burned in an engine. the total mass of co, co2, and h2o produced is 11.53 kg. calculate the efficiency of the process; that is, calculate the fraction of octane converted to co2. the density of octane is 2.650 kg/gal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 23.06.2019 02:00
The bone of a dinosaur and the imprint of a leaf are examples of which kind of fossils? a) index b) body c) amber d) trace
Answers: 1
You know the right answer?
Octane (c8h18) is a component of gasoline. complete combustion of octane yields h2o and co2. incompl...
Questions

Chemistry, 22.12.2019 02:31






Mathematics, 22.12.2019 02:31

Mathematics, 22.12.2019 02:31



Mathematics, 22.12.2019 02:31





Mathematics, 22.12.2019 02:31


Mathematics, 22.12.2019 02:31

Physics, 22.12.2019 02:31

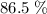 of octane had been converted to carbon dioxide CO₂.
of octane had been converted to carbon dioxide CO₂.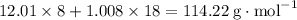
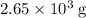 , which corresponds to
, which corresponds to 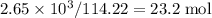 of octane.
of octane.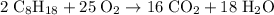
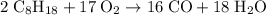
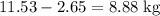 heavier than that of the octane supplied. Thus
heavier than that of the octane supplied. Thus 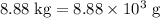 of oxygen were consumed in the combustion. There are
of oxygen were consumed in the combustion. There are 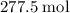 of oxygen molecules in
of oxygen molecules in 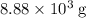 of oxygen.
of oxygen. (
(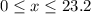 ). The number of moles of octane that had undergone incomplete combustion through the second equation would thus equal
). The number of moles of octane that had undergone incomplete combustion through the second equation would thus equal 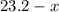 .
.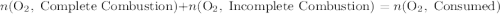
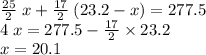
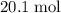 out of the 23.2 moles of octane had undergone complete combustion to produce carbon dioxide.
out of the 23.2 moles of octane had undergone complete combustion to produce carbon dioxide.