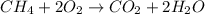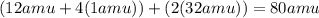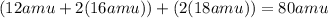Chemistry, 05.07.2019 20:00 keishlav5183
Describe the relationship of the atoms shown by: ch4(g) + 2o2(g) → co2(g) + 2h2o(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1
You know the right answer?
Describe the relationship of the atoms shown by: ch4(g) + 2o2(g) → co2(g) + 2h2o(g)...
Questions


Mathematics, 17.02.2021 22:20

Mathematics, 17.02.2021 22:20

Spanish, 17.02.2021 22:20

Spanish, 17.02.2021 22:20

Mathematics, 17.02.2021 22:20

History, 17.02.2021 22:20

Chemistry, 17.02.2021 22:20

English, 17.02.2021 22:20



Mathematics, 17.02.2021 22:20

Biology, 17.02.2021 22:20

Mathematics, 17.02.2021 22:20

Arts, 17.02.2021 22:20



Biology, 17.02.2021 22:20

Mathematics, 17.02.2021 22:20