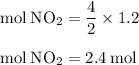Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 01:30
If a particle has z = 25 and 23 electrons, what is its charge?
Answers: 2
You know the right answer?
For the reaction shown, calculate how many moles of no2 form when each amount of reactant completely...
Questions


Mathematics, 09.04.2021 01:30

Mathematics, 09.04.2021 01:30

Physics, 09.04.2021 01:30


Mathematics, 09.04.2021 01:30


History, 09.04.2021 01:30





Mathematics, 09.04.2021 01:30

Chemistry, 09.04.2021 01:30

Biology, 09.04.2021 01:30

Mathematics, 09.04.2021 01:30

Mathematics, 09.04.2021 01:30



Mathematics, 09.04.2021 01:30