
Chemistry, 06.07.2019 05:30 crawford184232323234
Asample of an unknown compound contains 82.63% c, and 17.37% h. determine the empirical formula.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2


Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
Asample of an unknown compound contains 82.63% c, and 17.37% h. determine the empirical formula....
Questions








Mathematics, 15.07.2020 20:01




English, 15.07.2020 20:01




Social Studies, 15.07.2020 20:01

Geography, 15.07.2020 20:01

Computers and Technology, 15.07.2020 20:01


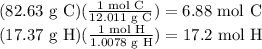
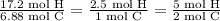
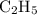 .
.

