
Chemistry, 06.07.2019 13:00 briannagotfanz
Acompound is used as a food additive. the compound has a molar mass of 176.124 grams/mole. a 692.5-gram sample undergoes decomposition, producing 283.4 grams of carbon, 31.7 grams of hydrogen, and 377.4 grams of oxygen. what is the molecular formula of the compound?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 09:00
Which of the following are in a chemical family a. ca, sc, k b. cu, ag, au c. so, ge, sb
Answers: 1

Chemistry, 23.06.2019 10:10
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3

Chemistry, 23.06.2019 17:30
Two examples of energy transformations are shown. the energy transformations are similar because they both involve transformations that begin with chemical energy. begin with electrical energy. result in radiant energy. result in mechanical energy.
Answers: 2
You know the right answer?
Acompound is used as a food additive. the compound has a molar mass of 176.124 grams/mole. a 692.5-g...
Questions

English, 23.03.2021 23:10


Biology, 23.03.2021 23:10

History, 23.03.2021 23:10

Mathematics, 23.03.2021 23:10


Mathematics, 23.03.2021 23:10

Mathematics, 23.03.2021 23:10

Mathematics, 23.03.2021 23:10





Mathematics, 23.03.2021 23:10



Mathematics, 23.03.2021 23:10



Mathematics, 23.03.2021 23:10

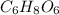 .
.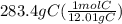
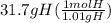
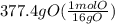
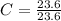 = 1
= 1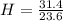 = 1.33
= 1.33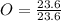 = 1
= 1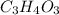 .
.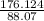 = 2
= 2

