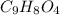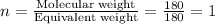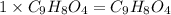Chemistry, 07.07.2019 02:30 jesh0975556
Aspirin has a molar mass of 180g/mol. if the empirical formula is c9h8o4, what is the molecular formula of aspirin ? fill in the blanks for the subscripts of the formula below. you have to have a whole number subscript for each blank even if it is a 1. c__h__o__


Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

You know the right answer?
Aspirin has a molar mass of 180g/mol. if the empirical formula is c9h8o4, what is the molecular form...
Questions


Chemistry, 25.01.2021 18:40

Biology, 25.01.2021 18:40

Mathematics, 25.01.2021 18:40

Mathematics, 25.01.2021 18:40

Mathematics, 25.01.2021 18:40



Mathematics, 25.01.2021 18:40

Mathematics, 25.01.2021 18:40

Mathematics, 25.01.2021 18:40



Mathematics, 25.01.2021 18:40


Mathematics, 25.01.2021 18:40




Social Studies, 25.01.2021 18:40