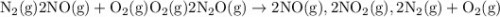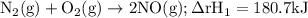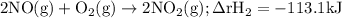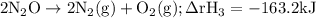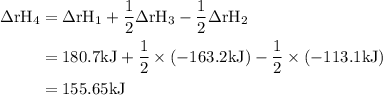Chemistry, 07.07.2019 02:30 villarrealc1987
Given the following data: n2(g)2no(g)++o2(g)o2(g)2n2o(g)→→→2n o(g),2no2(g),2n2(g)+o2(g),δh=+180.7 kjδh=−113.1kjδh=−163.2kj use hess's law to calculate δh for the following reaction: n2o(g)+no2(g)→3no(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
Given the following data: n2(g)2no(g)++o2(g)o2(g)2n2o(g)→→→2n o(g),2no2(g),2n2(g)+o2(g),δh=+180.7 k...
Questions


Chemistry, 12.02.2022 23:00

Mathematics, 12.02.2022 23:00




Computers and Technology, 12.02.2022 23:00

Social Studies, 12.02.2022 23:00

Biology, 12.02.2022 23:00


Geography, 12.02.2022 23:00


English, 12.02.2022 23:00

English, 12.02.2022 23:00

Mathematics, 12.02.2022 23:00


Mathematics, 12.02.2022 23:00