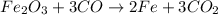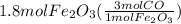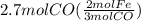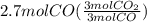Read the chemical equation. fe2o3 + co → fe + co2 if 1.8 moles of fe2o3 react with 2.7 moles of co, how many moles of each product are formed? 5.4 moles fe and 1.8 moles co2 2.7 moles fe and 0.9 moles co2 3.6 moles fe and 5.4 moles co2 1.8 moles fe and 2.7 moles co2

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
Read the chemical equation. fe2o3 + co → fe + co2 if 1.8 moles of fe2o3 react with 2.7 moles of co,...
Questions




Mathematics, 07.07.2019 11:00



English, 07.07.2019 11:00



Biology, 07.07.2019 11:00

Spanish, 07.07.2019 11:00

Computers and Technology, 07.07.2019 11:00



Biology, 07.07.2019 11:00

History, 07.07.2019 11:00

Geography, 07.07.2019 11:00

Biology, 07.07.2019 11:00


History, 07.07.2019 11:00

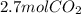 .
.