
Chemistry, 07.07.2019 04:30 Tyrant4life
Type the correct answer in the box. express your answer to three significant figures. this balanced equation shows the reaction of sodium hydroxide and sulfuric acid: 2naoh + h2so4 → na2so4 + 2h2o. in a laboratory experiment, a student mixes 355 grams of sulfuric acid with an excess of sodium hydroxide. what is the theoretical mass of sodium sulfate produced? refer to the periodic table and the polyatomic ion resource. the theoretical mass of sodium sulfate is grams.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2
You know the right answer?
Type the correct answer in the box. express your answer to three significant figures. this balanced...
Questions


Mathematics, 15.01.2021 19:40

Mathematics, 15.01.2021 19:40

History, 15.01.2021 19:40

Mathematics, 15.01.2021 19:40


Mathematics, 15.01.2021 19:40

Mathematics, 15.01.2021 19:40


Computers and Technology, 15.01.2021 19:40

Business, 15.01.2021 19:40

Mathematics, 15.01.2021 19:40



Mathematics, 15.01.2021 19:40


Mathematics, 15.01.2021 19:40

History, 15.01.2021 19:40


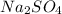 ) is 514.118 grams.
) is 514.118 grams.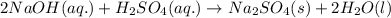
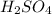 is a limiting reagent as the quantity of the product will depend on it.
is a limiting reagent as the quantity of the product will depend on it.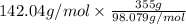 of
of 

