
Chemistry, 07.07.2019 15:00 gdominguez2005
H+ ions increase in concentration at lower ph values. calculate how many more h+ ions there are in a solution at a ph = 2 than in a solution at a ph = 6. find the concentration of h+ ions at a ph = 2 and at a ph = 6 in table b. then divide the concentration of h+ ions at a ph = 2 by the of h+ ions at a ph = 6. record your answer in table c. what is the concentration of h+ ions at a ph = 2? mol/l what is the concentration of h+ ions at a ph = 6? mol/l how many more h+ ions are there in a solution at a ph = 2 than in a solution at a ph = 6? need asap give brainliest

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1
You know the right answer?
H+ ions increase in concentration at lower ph values. calculate how many more h+ ions there are in a...
Questions





History, 06.05.2020 05:57

Mathematics, 06.05.2020 05:57

History, 06.05.2020 05:57



History, 06.05.2020 05:57







Social Studies, 06.05.2020 05:57



![pH=-log[H⁺]](/tpl/images/0062/1602/22ab6.png)
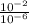 = 10⁴
= 10⁴


