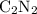Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1

You know the right answer?
Atoxic gas. a, consists of 53.8% nitrogen and 46.2% carbon by mass. at 273 k and 1.01 x 10^5 pa, 1.0...
Questions

English, 21.04.2021 19:20

Mathematics, 21.04.2021 19:20


English, 21.04.2021 19:20

French, 21.04.2021 19:20

Mathematics, 21.04.2021 19:20

Mathematics, 21.04.2021 19:20

Mathematics, 21.04.2021 19:20


Mathematics, 21.04.2021 19:20


Mathematics, 21.04.2021 19:20


Social Studies, 21.04.2021 19:20

Mathematics, 21.04.2021 19:20

Mathematics, 21.04.2021 19:20

Biology, 21.04.2021 19:20

Mathematics, 21.04.2021 19:20


Mathematics, 21.04.2021 19:20

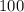 grams of the sample would contain
grams of the sample would contain 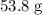 of nitrogen, and
of nitrogen, and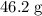 of carbon.
of carbon.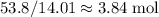 of nitrogen atoms, and
of nitrogen atoms, and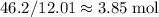 of carbon atoms.
of carbon atoms.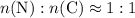 and the empirical formula
and the empirical formula 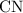 .
.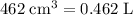 sample:
sample: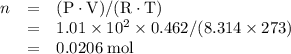
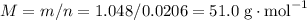
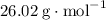 , formula mass for the empirical formula
, formula mass for the empirical formula