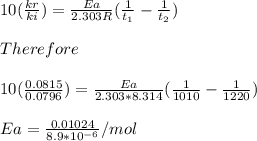Chemistry, 07.07.2019 21:00 madisontrosclair2
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollution generated in automobile engines. the decomposition of nitric oxide (no) to n2 and o2 is second order with a rate constant of 0.0796 m−1⋅s−1 at 737∘c and 0.0815 m−1⋅s−1 at 947∘c. you may want to reference (page) section 14.5 while completing this problem. part a calculate the activation energy for the reaction. express the activation energy in kilojoules per mole to three significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 05:50
Which of the following is not a characteristic of s waves?
Answers: 1
You know the right answer?
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollutio...
Questions





Mathematics, 09.10.2019 03:00

Social Studies, 09.10.2019 03:00

Social Studies, 09.10.2019 03:00

English, 09.10.2019 03:00


Mathematics, 09.10.2019 03:00

Biology, 09.10.2019 03:00


Biology, 09.10.2019 03:00





Social Studies, 09.10.2019 03:00

Arts, 09.10.2019 03:00