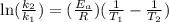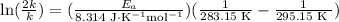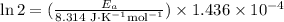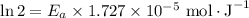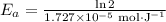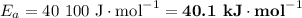Chemistry, 07.07.2019 22:00 gonzalesalexiaouv1bg
If a temperature increase from 10.0 ∘c to 22.0 ∘c doubles the rate constant for a reaction, what is the value of the activation barrier for the reaction? \

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1


Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
You know the right answer?
If a temperature increase from 10.0 ∘c to 22.0 ∘c doubles the rate constant for a reaction, what is...
Questions

Mathematics, 01.02.2021 03:00

History, 01.02.2021 03:00

Chemistry, 01.02.2021 03:00

Mathematics, 01.02.2021 03:00

Mathematics, 01.02.2021 03:00


Arts, 01.02.2021 03:00

Social Studies, 01.02.2021 03:00



History, 01.02.2021 03:00

Social Studies, 01.02.2021 03:00



Biology, 01.02.2021 03:00

Mathematics, 01.02.2021 03:00


Business, 01.02.2021 03:00

Social Studies, 01.02.2021 03:00

Mathematics, 01.02.2021 03:00