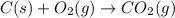The question: a new grill has a mass of 30.0 kg. you put 2.0 kg of charcoal in the grill. you burn all the charcoal and the grill has a mass of 30.0 kg. what is the mass in kg of the gases given off? (assume that the charcoal is pure carbon solid and that it burns completely in oxygen). the mass of carbon burnt = 2kg = 2000g moles of carbon burnt = 2000/12 => moles of co2 produced = 2000/12 => mass of co2 produced = 2000/12*44 = 2 333.33 g = 2.33 kg in the answer, where did the 44 come from?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
The question: a new grill has a mass of 30.0 kg. you put 2.0 kg of charcoal in the grill. you burn a...
Questions

Mathematics, 17.04.2021 04:40

Mathematics, 17.04.2021 04:40


Mathematics, 17.04.2021 04:40

Mathematics, 17.04.2021 04:40

Mathematics, 17.04.2021 04:40

Mathematics, 17.04.2021 04:40

Social Studies, 17.04.2021 04:40

History, 17.04.2021 04:40

Health, 17.04.2021 04:40

Mathematics, 17.04.2021 04:40


Mathematics, 17.04.2021 04:40

Advanced Placement (AP), 17.04.2021 04:40


Mathematics, 17.04.2021 04:40

Mathematics, 17.04.2021 04:40

History, 17.04.2021 04:40

Chemistry, 17.04.2021 04:40

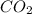 .
.