

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

You know the right answer?
Calculate the number of moles of bromine present in 20.5 ml of br2(l), whose density is 3.12 g/ml....
Questions





Mathematics, 08.04.2021 18:50

English, 08.04.2021 18:50

Mathematics, 08.04.2021 18:50


English, 08.04.2021 18:50


Mathematics, 08.04.2021 18:50


Mathematics, 08.04.2021 18:50

Mathematics, 08.04.2021 18:50





Chemistry, 08.04.2021 18:50

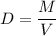
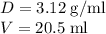
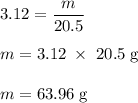
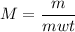
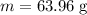
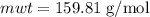

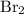 present in 20.5 ml has been 0.4 mol.
present in 20.5 ml has been 0.4 mol.

