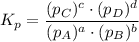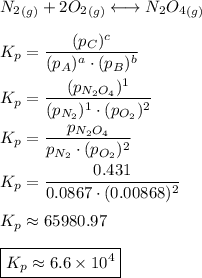Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Supongamos que estás estudiando dos estrellas. ambas estrellas tienen la misma magnitud aparente, pero la estrella a tiene una magnitud absoluta mayor que la estrella b. ¿que puedes decir acerca de la distancia a la tierra de estas dos estrellas?
Answers: 3

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1
You know the right answer?
Consider the equilibrium, n2(g) + 2o2(g) < > n2o4(g). calculate the equilibrium constant, kpi...
Questions



Mathematics, 25.11.2020 03:00



Mathematics, 25.11.2020 03:00


Mathematics, 25.11.2020 03:00

Computers and Technology, 25.11.2020 03:00

Mathematics, 25.11.2020 03:00


Mathematics, 25.11.2020 03:00

Engineering, 25.11.2020 03:00






Mathematics, 25.11.2020 03:00

Mathematics, 25.11.2020 03:00