Chemistry, 08.07.2019 11:00 Brainly264
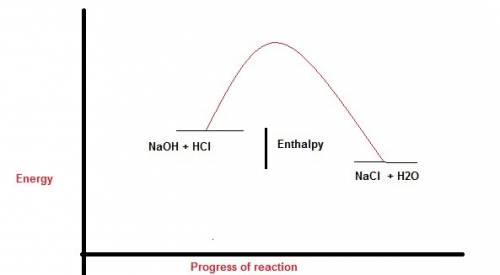
Perhatikan persamaan termokimia berikut. hcl (aq) + naoh (aq) -> nacl (aq) + h2o (l). delta h=-54 kj a. gambarlah diagram tingkat energi untuk reaksi tersebut b. berapakah perubahan entalpi jika 100 ml naoh 1 m ? c. berapakah perubahan entalpi jika 10 ml hcl 1 m direaksikan dengan 20 ml naoh 1 m ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2
You know the right answer?
Perhatikan persamaan termokimia berikut. hcl (aq) + naoh (aq) -> nacl (aq) + h2o (l). delta h=-5...
Questions