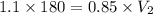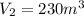Chemistry, 08.07.2019 17:30 kamelyawarren1
Suppose that on a hot and sticky afternoon in the spring, a tornado passes over the high school. if the air pressure in the lab (volume of 180 m3) was 1.1 atm before the storm and 0.85 atm during the storm, to what volume would the laboratory try to expand in order to make up for the large pressure difference outside? 190 m3 230 m3 1,800 m3 7,100 m3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

You know the right answer?
Suppose that on a hot and sticky afternoon in the spring, a tornado passes over the high school. if...
Questions

English, 20.10.2020 14:01



Mathematics, 20.10.2020 14:01


History, 20.10.2020 14:01


Mathematics, 20.10.2020 14:01


Chemistry, 20.10.2020 14:01

Mathematics, 20.10.2020 14:01


Mathematics, 20.10.2020 14:01

Physics, 20.10.2020 14:01

Physics, 20.10.2020 14:01


History, 20.10.2020 14:01

English, 20.10.2020 14:01

Arts, 20.10.2020 14:01

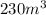
 (At constant temperature and number of moles)
(At constant temperature and number of moles)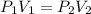
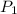 = initial pressure of gas = 1.1 atm
= initial pressure of gas = 1.1 atm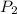 = final pressure of gas = 0.85 atm
= final pressure of gas = 0.85 atm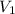 = initial volume of gas =
= initial volume of gas = 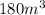
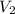 = final volume of gas = ?
= final volume of gas = ?