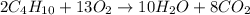Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

You know the right answer?
Disposable lighters contain liquid butane (c4h10) that is vaporized and then burned to produce co2 a...
Questions

Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00

Chemistry, 11.12.2021 01:00


Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00


Mathematics, 11.12.2021 01:00

Mathematics, 11.12.2021 01:00


Physics, 11.12.2021 01:00

History, 11.12.2021 01:00




Chemistry, 11.12.2021 01:00

Chemistry, 11.12.2021 01:00

Chemistry, 11.12.2021 01:00

History, 11.12.2021 01:00

Physics, 11.12.2021 01:00

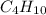 ) is a hydrocarbon used in disposable lighters. It undergoes a combustion reaction to produce a flammable gas.
) is a hydrocarbon used in disposable lighters. It undergoes a combustion reaction to produce a flammable gas.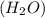 and carbondioxide (
and carbondioxide (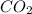 ). It is a exothermic reaction which means that heat is released during this reaction.
). It is a exothermic reaction which means that heat is released during this reaction.