
Chemistry, 08.07.2019 21:00 khadythiam6957
When solid phosphorus (p4) reacts with oxygen gas, diphosphorus pentoxide is formed. initially 2.87 mol of phosphorus and 3.86 mol of oxygen are combined. (assume 100% yield). a) write a balanced equation for the reaction. b) what is the limiting reactant? c) how many miles of excess reactant remain after the reaction is complete?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 23:10
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium.b)heavier than helium.c)the same weight as helium.d)dependent on the element that reacted with carbon.
Answers: 3

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
When solid phosphorus (p4) reacts with oxygen gas, diphosphorus pentoxide is formed. initially 2.87...
Questions



Social Studies, 20.01.2020 23:31











Mathematics, 20.01.2020 23:31

Computers and Technology, 20.01.2020 23:31




Social Studies, 20.01.2020 23:31

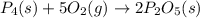
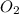 .
.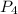 is 0.94 moles.
is 0.94 moles.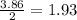 moles of
moles of 


